| Recently, Asatekin and Gleason from MIT reported on their work using conformal initiated chemical vapor deposition (iCVD) to control the size and surface functionality of polymeric nanopores. Their work demonstrates a simple, scalable nanomanufacturing process to synthesize nanopore membranes for separation of small molecules. Utilizing a facile approach to tune the pore size, shape, and surface chemical properties, an approach to customize membrane properties for a range of separations applications has been developed. Additional models have been developed by the authors to better understand the conformal deposition processes for ultra-high aspect ratio nanopores. These models will be instrumental for further investigations of the range of monomers and initiators that can be used for the iCVD process. |
Reviewed by Jeff Morse, PhD, National Nanomanufacturing Network
- Asatekin A, Gleason KK. 2011. Polymeric Nanopore Membranes for Hydrophobicity-Based Separations by Conformal Initiated Chemical Vapor Deposition. Nano Letters 11:677-686. doi:10.1021/nl103799d
The use of porous membranes provides a scalable, low cost energy efficient approach to industrial scale separations, such as size-based sieving or desalination by reverse osmosis.Membrane separation using these techniques is particularly challenging for the case of small molecules of similar size ranges. For biological and pharmaceutical applications, these approaches to separations become important as industrial scale techniques are required to replace present methods such as chromatography. Additionally, molecular fractionation can also occur during sieving operation. Membranes that can enable separations based on other chemical properties, for instance hydrophobicity, could extend the range of applications using these techniques. In order to achieve such membrane performance, the pore diameters need to be similar in size to the molecules being separated, with controlled surface composition and functionality. In this manner the permeation of the molecules through the pore is dominated by interactions of the molecule with the pore wall, thus solutes that adsorb more strongly to the pore surface are partitioned more into the pore and permeate more rapidly. Thus separation based on both size and chemical structure can be achieved. Previous approaches to achieve such pore structures have utilized atomic layer deposition (ALD) of silicon dioxide to narrow the pores of anodized aluminum oxide (AAO) membranes, then functionalized the pore surface using silane. Similarly, nanotubes were formed by electroless deposition of gold within an AAO template and subsequently functionalized with a self-assembled monolayer (SAM). While these techniques offer a means to controllably tune the diameter of the nanopore down to the few nanometers range, and subsequently control the chemical properties of the pore surface, the synthesis processes require high temperatures. Additionally, the processes may not be environmentally friendly, and are costly to scale. A scalable, low cost method of forming nanopore membranes with controlled surface chemistry is necessary to address present and future industrial needs.
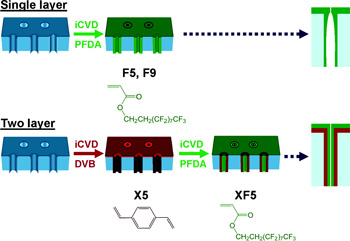
In order to characterize the permeation properties and separation selectivity of the hydrophobic nanopore membrane structures, two sets of similar sized small molecules were selected with different levels of hydrophobicity. Comparison of the results for the different nanopore structures illustrated that the two layer conformally coated nanopores exhibited the highest diffusion selectivity, ranging from 2-4 times greater than that of the single layer nanopores having the same diameter. Furthermore, to illustrate the impact of hydrophobicity on diffusion selectivity, the two layer conformal nanopore structures exhibited 10-25 times greater selectivity than the nanopore membrane with only the pDVB coating. Thus a simple, scalable nanomanufacturing process has been demonstrated to synthesize nanopore membranes for separation of small molecules. Utilizing a facile approach to tune the pore size, shape, and surface chemical properties, an approach to customize membrane properties for a range of separations applications has been developed. Additional models have been developed by the authors to better understand the conformal deposition processes for ultra-high aspect ratio nanopores. These models will be instrumental for further investigations of the range of monomers and initiators that can be used for the iCVD process.
Figure reprinted with permission from Asatekin A, Gleason KK. 2011. Polymeric Nanopore Membranes for Hydrophobicity-Based Separations by Conformal Initiated Chemical Vapor Deposition. Nano Letters 11:677-686. doi:10.1021/nl103799d Copyright 2011 American Chemical Society.
