The cement industry is one of the largest sources worldwide of carbon emissions, accounting for around five per cent of global emissions. Two thirds of these CO2 emissions are released during the chemical process of burning limestone for cement production and can only be cut by extracting the CO2 from the emissions in one form or another.
In a previous Nanowerk Spotlight we looked at how nanoengineering of cement-based materials can result in outstanding or smart properties ("Nanotechnology in the cement industry – a patent analysis").
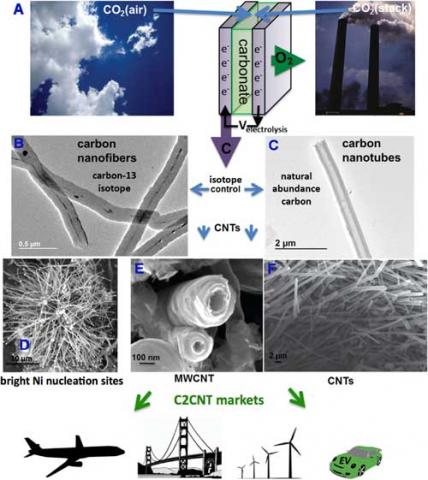
Now, in two new studies, researchers show that cement plants can have their carbon dioxide exhaust eliminated while co-producing carbon nanotubes.
"We have demonstrated that with our C2CNT (carbon dioxide into carbon nanotubes) process, a wide portfolio of tailored carbon nanotubes (CNTs), such as those with special shapes or conductivity can be made," Stuart Licht, a professor of chemistry at George Washington University, tells Nanowerk.
In the first paper, in Journal of CO2 Utilization ("Transformation of the greenhouse gas CO2 by molten electrolysis into a wide controlled selection of carbon nanotubes"), Licht and his team present the transformation of CO2 into specific, controlled carbon nanotubes.
This study presents a straightforward process that transforms CO2 to carbon nanotubes by molten electrolysis with inexpensive (nickel and steel) electrodes and low voltage. This synthesis consumes only CO2 and electricity, and is constrained only by the cost of electricity.
"Control of electrolysis parameters opens up a wide portfolio of CNT morphologies including hollow or solid, thick or thin walled," says Licht. "Our portfolio also includes doped CNT. Molten carbonate electrosynthesized boron doped CNTs have high electrical conductivity. The CNT from CO2 synthesis remains single step (one pot) in which a specific impurity is dissolved in the electrolyte to achieve a desired doped CNT characteristic."
The C2CNT technology is applicable to the direct removal of atmospheric CO2 or the elimination of industrial CO2 from smokestacks.
In the second paper, also in the Journal of CO2 Utilization ("Co-production of cement and carbon nanotubes with a carbon negative footprint"), Licht presents the use of C2CNT to retrofit cement plants.
Cement production today has a massive carbon footprint and simultaneously releases CO2 both from limestone and from fossil fuels, and hence cement plant smokestacks have much higher CO2 content (5 times higher than gas fired electric power plants).
This study compares conventional cement plants to alternative C2CNT cement plants and shows:
- In the C2CNT cement plant CNTs are produced by molten carbonate electrolysis of CO2.
- The conventional cement plant emits 1.1 ton of CO2 per ton cement produced.
- The C2CNT cement plant emits no CO2, converting it to a valuable CNT co-product.
Per ton CO2 avoided, the C2CNT cement plant consumes $50 electricity, emits no CO2, and produces $100 worth of cement and ∼$60,000 worth of CNTs.
Scaling up the C2CNT process to work with commercial plants is the ongoing second stage of the researchers' investigations. As part of this effort they have advanced to the semifinals of the Carbon XPrize as the C2CNT team. The Carbon XPrize is a global competition for research teams to transform CO2 from a power plant into the most valuable product.
References:
"Transformation of the greenhouse gas CO2 by molten electrolysis into a wide controlled selection of carbon nanotubes" Jiawen Ren, Marcus Johnson, Richa Singhal, Stuart Licht. Journal of CO2 Utilization. Vol 18, March 2017, Pages 335-344. doi:10.1016/j.jcou.2017.02.005
"Co-production of cement and carbon nanotubes with a carbon negative footprint" Stuart Licht. Journal of CO2 Utilization. Vol 18, March 2017, Pages 378-389. doi:10.1016/j.jcou.2017.02.011
Source: Nanowerk